
| Strong, beautiful and light | The development of aluminium as a product |
History of discovery
1807 – 1825 – 1845

The English chemist Sir Humphrey Davy underlined the existence of the element arguing that “alum” was the salt of an unknown metal which he said should be called alumium. The name was respelt as the more pleasant sounding aluminium by later scientists. Davy tried unsuccessfully to produce aluminium by electrolysing a fused mixture of aluminium oxide and potash.
Following Davy’s work the Danish physicist H.C. Oersted managed to produce the first nodules of aluminium by heating potassium amalgam with aluminium.
Friedrich Whler in Germany established many of the metal’s properties, including the remarkable lightness. It was the discovery of this property that truly excited researchers and paved the way for more generous development funding.
1854
The Frenchman Henri Sainte-Claire Deville developed a reduction process using sodium which, with further refinement by others, allowed the production of high cost metal in limited quantities and his process was copied throughout Europe. Scientists were now in the position to produce kilograms rather than mere grams an important step towards the industrial use of aluminium.
1886
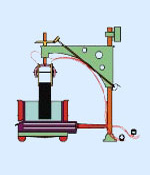
The smelting process that is still used today was discovered almost simultaneously but independently in the United States and France. Working in a woodshed in Ohio, Charles Martin Hall made the same discovery that metallurgist Paul Lois Toussaint Hroult made in a makeshift laboratory in Gentilly: both men dissolved aluminium oxide in molten cryolite and then extracted the aluminium by electrolysis. Hall filed patents in the USA and Hroult in France but Hall was eventually credited with being the earlier inventor due to the slightly earlier patent application that he made.
1888
| The success of the Hall/Hroult process was advanced when Karl Bayer, an Austrian, invented an improved process for making aluminium oxide from bauxite. These inventions sealed the fate of aluminium by 1890 the cost of aluminium had tumbled some 80 percent from Deville’s prices. The metal was now a commercial commodity, how would it be used? |
Picture: Héroult’s electrolytic cell used for producing aluminium electrolytically from aluminium oxide (alumina) dissolved in cryolite.