More than 160 million tonnes of bauxite are mined each year. The major locations of deposites are found in tropic and sub-tropic areas. Bauxite is currently being extracted in Australia, Central and South America (Jamaica, Brazil, Surinam, Venezuela, Guyana), Africa (Guinea), Asia (India, China), Russia (and Kazakhstan) and Europe (Greece).
 |
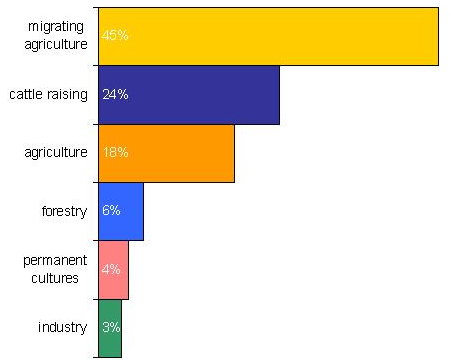
The material is mainly extracted by open-cast mining, which has a variable and highly site-specific effect on the local environment. The primary ecological concerns connected to this operation are related to the clearing of vegetation, affect on local flora and fauna, and soil erosion. Also, the conservation of rain forests is a key concern often voiced with regard to bauxite mining. Only about 15% of the world’s bauxite mining is today conducted in tropical rain forests. The area affected by bauxite mining is about 160m²/kt.
|
Alumina production
 |
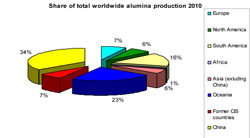
The aluminium hydroxide is then precipitated from the soda solution, washed and dried while the soda solution is recycled. After calcination, the end-product, aluminium oxide (Al2O3), is a fine grained white powder. Four tonnes of bauxite are required to produce two tonnes of alumina which in turn produces one tonne of aluminium at the primary smelter. In 2010, 87.4mil tonnes of alumina were produced world-wide. Alumina refineries are often located near to bauxite mines for logistics reasons. |
Electrolysis
Aluminium primary smelting and casting
Primary aluminium is produced in reduction plants (or “smelters”), where pure aluminium is extracted from alumina by the Hall-Héroult process. The reduction of alumina into liquid aluminium is operated at around 950 degrees Celsius in a cryolite bath under high intensity electrical current. This process takes place in electrolytic cells (or “pots”), where carbon cathodes form the bottom of the pot and act as the negative electrode. Anodes (positive electrodes) are held at the top of the pot and are consumed during the process when they react with the oxygen coming from the alumina. There are two types of anodes currently in use. All potlines built since the early 1970s use the prebake anode technology, where the anodes, manufactured from a mixture of petroleum coke and coal tar pitch (acting as a binder), are pre-baked in separate anode plants. In the Soederberg technology, the carbonaceous mixture is fed directly into the top part of the pot, where self-baking anodes are produced using the heat released by the electrolytic process.
At regular intervals, molten aluminium tapped from the pots is transported to the cast house where it is alloyed in holding furnaces by the addition of other metals (according to the users needs), cleaned of oxides and gases, and then cast into ingots. These can take the form of extrusion billets, for extruded products, or rolling ingots, for rolled products, depending on the way it is to be further processed. Aluminium mould castings are produced by foundries which use this technique to manufacture shaped components.
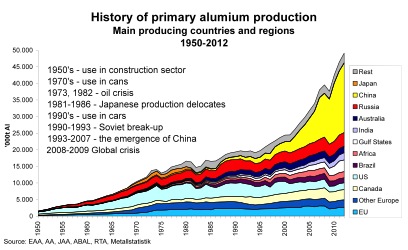 |
World-wide trends in production are shown in the following graph. Aluminium output has increased by a factor of 13 since 1950, making aluminium the most widely used non-ferrous metal. In 2010, the world-wide production of primary aluminium was about 42.6 million tonnes per year for and installed capacity of 53.6 million tonnes. Very recently China developped its aluminium production very rapidly, and it is the biggest producer in the world with over 17 million tonnes of production. The other main production areas are North America, Europe (4.2 Mt), former Cis, Africa, Australia, Brazil, India, and the Middle East. In Europe the main producing countries are Germany, France, Spain, Norway and Iceland. World-wide, production plants are mainly located where suitable electrical energy resources are available. Source: EAA, AA, JAA, ABAL, Alcan, Metallstatistik |